AliveCor
By MacRumors Staff
AliveCor Articles

Apple Watch Won't Face Import Ban as Apple Wins AliveCor ITC Battle
Apple today scored another victory in the ongoing lawsuit that AliveCor levied against it in 2021, with the federal appeals court confirming the invalidation of three patents that AliveCor claimed Apple violated with the Apple Watch. As a result, the court has vacated an ITC ruling that could have led to an Apple Watch import ban.
In a statement to MacRumors, Apple thanked the court for its...
Read Full Article 45 comments

Apple Won't Face AliveCor Antitrust Lawsuit Over Apple Watch Heart Rate Technology
An antitrust lawsuit that AliveCor filed against Apple back in 2021 will not proceed, with the judge overseeing the case today filing a summary judgment in Apple's favor.
The full ruling is under seal as of now due to confidentiality requests from Apple and AliveCor, but the filing makes it clear that the case went in Apple's favor and the Cupertino company was not found to have engaged in...

AliveCor Praises Apple Watch Ban Amid Its Own Health Tech Patent Dispute With Apple
Health technology company AliveCor has praised the International Trade Commission (ITC)'s ban on sales of the Apple Watch Series 9 and Apple Watch Ultra 2 in the United States.
AliveCor, like Masimo, accuses Apple of violating its patents related to heath technology used in the Apple Watch. While Masimo's fight focuses on the Apple Watch's blood oxygen sensing capabilities, AliveCor contends ...

Apple Watch Risks US Import Ban After Biden Administration Upholds Patent Ruling
The Biden administration has declined to overrule a U.S. International Trade Commission decision that the Apple Watch infringes patents from medical device company AliveCor, potentially paving the way for an import ban on Apple's smartwatch depending on how the appeals process pans out.
California-based AliveCor said in a statement that it was informed the Biden administration would not...
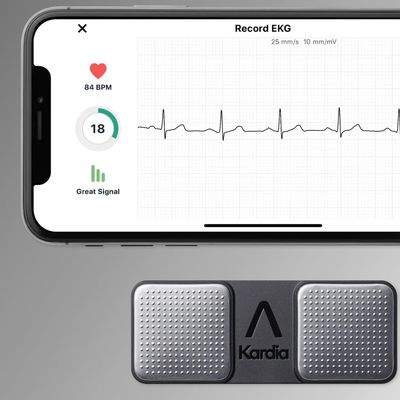
Apple Scores Win in AliveCor Legal Battle With USPTO Invalidating Several Patents
The United States Patent and Trademark Office's Patent Trial and Appeal Board today invalidated a trio of AliveCor patents that AliveCor used in a complaint with the International Trade Commission, which is a win for Apple. The patents all related to heart rate monitoring technology used in AliveCor products.
AliveCor in April 2021 filed a complaint with the ITC alleging that Apple had...

Apple Accuses AliveCor of 'Brazen' Patent Infringement in New Countersuit
Apple today filed a patent infringement lawsuit against AliveCor, a company that has developed the ECG "KardiaBand" designed for the Apple Watch, among other ECG-focused products. AliveCor and Apple are already in the midst of a legal battle following an ITC complaint and antitrust lawsuit that AliveCor filed last year
According to Apple, AliveCor's product line has not been successful with...

Apple to Face Claims it Bars Third-Party Heart-Rate App Functionality on Apple Watch
Apple must face claims it illegally monopolized the U.S. market for heart-rate monitoring apps on Apple Watch, a California-based federal judge said on Monday.
AliveCor, a company that that markets an ECG "KardiaBand" for the Apple Watch, filed an antitrust lawsuit against Apple in May 2021 accusing the Cupertino company of changing the heart-rate algorithm for the Apple Watch to gain an...

AliveCor Files Antitrust Suit Against Apple for Preventing Third-Party Irregular Heart Rhythm Analysis on Apple Watch
AliveCor, a company that that has developed an ECG 'KardiaBand' for the Apple Watch, today filed an antitrust lawsuit against Apple that accuses the Cupertino company of "monopolistic conduct."
According to AliveCor, Apple's decision to exclude third-party heart rate analysis providers from the Apple Watch has harmed AliveCor and impacted patients and consumers. To go along with the...

Study Suggests AliveCor KardiaBand for Apple Watch Can Be Used With AI Algorithm to Detect High Potassium
AliveCor, the company that makes an FDA-approved EKG band for the Apple Watch called KardiaBand, teamed up with the Mayo Clinic for a new study that suggests an AliveCor EKG device paired with artificial intelligence technology can non-invasively detect high levels of potassium in the blood.
A second study conducted by the Cleveland Clinic also confirms the KardiaBand's ability to accurately...

AliveCor 'Kardia Band' Medical Grade EKG Analyzer for Apple Watch Receives FDA Approval
Medical smartphone accessory company AliveCor this week received FDA-approval for its EKG Kardia Band, the first medical-grade accessory for Apple Watch. The band has been available in Europe for some months, but the product's clearance by the FDA means it can now be sold in the United States.
The Kardia Band for Apple Watch has an integrated metallic sensor in the strap that enables it to...












