iPhone Urinalysis App Draws U.S. Government Scrutiny
The U.S. Food and Drug Administration has sent a letter to BioSense Technologies over its iPhone uChek urinalysis system, asking why its medical app hasn't been cleared by the agency. The app is one of the first that turns the iPhone into a medical device, designed to read urinalysis test strips that are normally examined by users and compared to a color-coded chart.
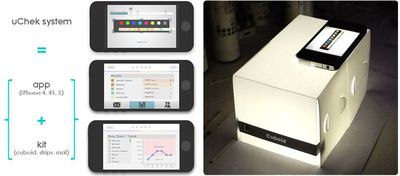
With the uChek system, patients can take a picture of the strip with the iPhone's camera and then receive an automated readout of parameters like glucose, urobilinogen, pH, ketone and more. The app also stores results which then can be analyzed over time.
Though medical device makers have adopted the iPhone for some measurements like blood glucose monitoring for diabetics, large scale use of smartphones and tablets as a replacement for existing medical devices has yet to take off -- likely due in large part to government regulation of medical devices.
From Bloomberg:
Biosense Technologies Private Ltd.’s uChek system isn’t cleared by the Food and Drug Administration and the agency said it wants to know why not, in a first-of-its-kind letter to a maker of a mobile-device application. The app relies on users, such as diabetics checking their glucose, to dip test strips in urine and use the smartphone’s camera to allow the system to processes and generate automated results.
UChek works with test strips made by Siemens AG (SIE) and Bayer AG (BAYN), which are only approved for visual reading and require new clearance for automated analysis, the FDA said in the letter. The agency has said it wants stricter rules for apps that directly diagnose or treat conditions, proposing in 2011 to apply similar quality standards as for heart stents, ultrasound machines and other medical devices.
The uChek kit can be purchased in the US and India for $40, while the uCheck iPhone app is a free download [Direct Link] from the App Store -- though the app can also manually read urine strips from other companies.
Popular Stories
Apple hasn't updated the AirPods Pro since 2022, and the earbuds are due for a refresh. We're counting on a new model this year, and we've seen several hints of new AirPods tucked away in Apple's code. Rumors suggest that Apple has some exciting new features planned that will make it worthwhile to upgrade to the latest model.
Subscribe to the MacRumors YouTube channel for more videos.
Heal...
Chase this week announced a series of new perks for its premium Sapphire Reserve credit card, and one of them is for a pair of Apple services.
Specifically, the credit card now offers complimentary annual subscriptions to Apple TV+ and Apple Music, a value of up to $250 per year.
If you are already paying for Apple TV+ and/or Apple Music directly through Apple, those subscriptions will...
Popular accessory maker Anker this month launched two separate recalls for its power banks, some of which may be a fire risk.
The first recall affects Anker PowerCore 10000 Power Banks sold between June 1, 2016 and December 31, 2022 in the United States. Anker says that these power banks have a "potential issue" with the battery inside, which can lead to overheating, melting of plastic...
In 2020, Apple added a digital car key feature to its Wallet app, allowing users to lock, unlock, and start a compatible vehicle with an iPhone or Apple Watch. The feature is currently offered by select automakers, including Audi, BMW, Hyundai, Kia, Genesis, Mercedes-Benz, Volvo, and a handful of others, and it is set to expand further.
During its WWDC 2025 keynote, Apple said that 13...
Apple's next-generation iPhone 17 Pro and iPhone 17 Pro Max are around three months away, and there are plenty of rumors about the devices.
Apple is expected to launch the iPhone 17, iPhone 17 Air, iPhone 17 Pro, and iPhone 17 Pro Max in September this year.
Below, we recap key changes rumored for the iPhone 17 Pro models:Aluminum frame: iPhone 17 Pro models are rumored to have an...
Apple last month announced the launch of CarPlay Ultra, the long-awaited next-generation version of its CarPlay software system for vehicles.
There was news this week about which automakers will and won't offer CarPlay Ultra, and we have provided an updated list below.
CarPlay Ultra is currently limited to newer Aston Martin vehicles in the U.S. and Canada. Fortunately, if you cannot...
Apple will finally deliver the Apple Watch Ultra 3 sometime this year, according to analyst Jeff Pu of GF Securities Hong Kong (via @jukanlosreve).
The analyst expects both the Apple Watch Series 11 and Apple Watch Ultra 3 to arrive this year (likely alongside the new iPhone 17 lineup, if previous launches are anything to go by), according to his latest product roadmap shared with...
Apple is planning to launch a low-cost MacBook powered by an iPhone chip, according to Apple analyst Ming-Chi Kuo.
In an article published on X, Kuo explained that the device will feature a 13-inch display and the A18 Pro chip, making it the first Mac powered by an iPhone chip. The A18 Pro chip debuted in the iPhone 16 Pro last year. To date, all Apple silicon Macs have contained M-series...