iPhone Urinalysis App Draws U.S. Government Scrutiny
The U.S. Food and Drug Administration has sent a letter to BioSense Technologies over its iPhone uChek urinalysis system, asking why its medical app hasn't been cleared by the agency. The app is one of the first that turns the iPhone into a medical device, designed to read urinalysis test strips that are normally examined by users and compared to a color-coded chart.
With the uChek system, patients can take a picture of the strip with the iPhone's camera and then receive an automated readout of parameters like glucose, urobilinogen, pH, ketone and more. The app also stores results which then can be analyzed over time.
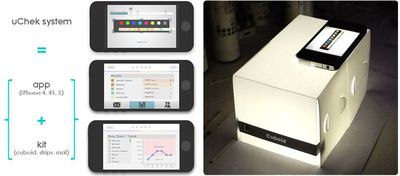
Though medical device makers have adopted the iPhone for some measurements like blood glucose monitoring for diabetics, large scale use of smartphones and tablets as a replacement for existing medical devices has yet to take off -- likely due in large part to government regulation of medical devices.
From Bloomberg:
Biosense Technologies Private Ltd.’s uChek system isn’t cleared by the Food and Drug Administration and the agency said it wants to know why not, in a first-of-its-kind letter to a maker of a mobile-device application. The app relies on users, such as diabetics checking their glucose, to dip test strips in urine and use the smartphone’s camera to allow the system to processes and generate automated results.
UChek works with test strips made by Siemens AG (SIE) and Bayer AG (BAYN), which are only approved for visual reading and require new clearance for automated analysis, the FDA said in the letter. The agency has said it wants stricter rules for apps that directly diagnose or treat conditions, proposing in 2011 to apply similar quality standards as for heart stents, ultrasound machines and other medical devices.
The uChek kit can be purchased in the US and India for $40, while the uCheck iPhone app is a free download [Direct Link] from the App Store -- though the app can also manually read urine strips from other companies.
Popular Stories
Apple today shared an ad that shows how the upgraded Center Stage front camera on the latest iPhones improves the process of taking a group selfie.
"Watch how the new front facing camera on iPhone 17 Pro takes group selfies that automatically expand and rotate as more people come into frame," says Apple. While the ad is focused on the iPhone 17 Pro and iPhone 17 Pro Max, the regular iPhone...
In select U.S. states, residents can add their driver's license or state ID to the Apple Wallet app on the iPhone and Apple Watch, and then use it to display proof of identity or age at select airports and businesses, and in select apps.
The feature is currently available in 13 U.S. states and Puerto Rico, and it is expected to launch in at least seven more in the future.
To set up the...
Apple is planning to launch new MacBook Pro models as soon as early March, but if you can, this is one generation you should skip because there's something much better in the works.
We're waiting on 14-inch and 16-inch MacBook Pro models with M5 Pro and M5 Max chips, with few changes other than the processor upgrade. There won't be any tweaks to the design or the display, but later this...
It has been a slow start to 2026 for Apple product launches, with only a new AirTag and a special Apple Watch band released so far. We are still waiting for MacBook Pro models with M5 Pro and M5 Max chips, the iPhone 17e, a lower-cost MacBook with an iPhone chip, long-rumored updates to the Apple TV and HomePod mini, and much more.
Apple is expected to release/update the following products...
Wednesday February 11, 2026 10:07 am PST by
Juli CloverApple today released iOS 26.3 and iPadOS 26.3, the latest updates to the iOS 26 and iPadOS 26 operating systems that came out in September. The new software comes almost two months after Apple released iOS 26.2 and iPadOS 26.2.
The new software can be downloaded on eligible iPhones and iPads over-the-air by going to Settings > General > Software Update.
According to Apple's release notes, ...